Research Update: CRISPR Gene Editing Proves Effective Three Years Into Trial
Individuals who have rare blood disorders that currently have no cure are keeping an eye on news coming out of the world of genetic engineering. When scientists gather to share their latest findings, it can be a source of hope for patients and their families who have been looking for some relief for their condition.
Attendees at the recent European Hematology Association Congress were the first to learn new results from a promising experiment that centers on CRISPR gene editing to combat rare diseases of a genetic origin.
The information is an update on one of the world’s longest-running experimental studies employing CRISPR, which began human trials back in 2019 to look for a cure for sickle cell disease and beta thalassemia, which are rare blood disorders for which we do not currently have a cure.
Harnessing CRISPR Gene Editing to Combat Genetic Disease
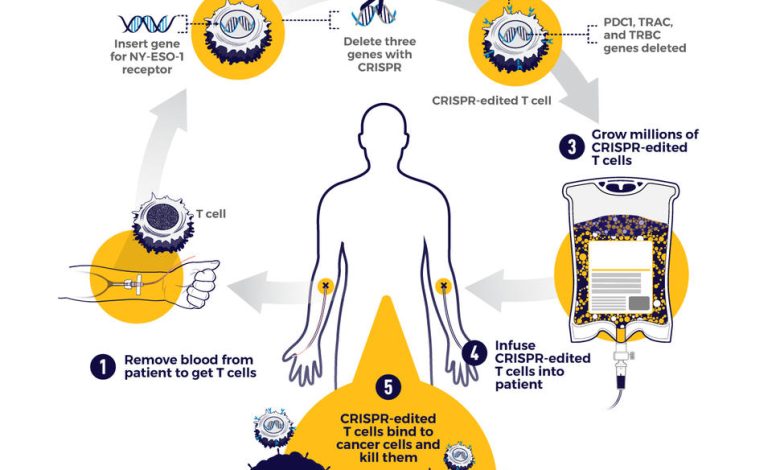
Clustered Regularly Interspaced Short Palindromic Repeats or CRISPR is a technology that scientists Sam Sternberg and Jennifer Doudna reported on in a ground-breaking study in Science in 2012. With CRISPR gene editing, you can actually make changes to a person’s cells and then reinfuse them into the body to tackle a disorder that has a genetic mutation.
Promising Results Using CRISPR Against Rare Disorders
Scientists from Vertex, a biotechnology company, launched human trials for their treatment based on CRISPR gene editing in 2019, aiming to cure beta thalassemia and sickle cell disease, which are disorders people inherit.
Using CRISPR, the researchers removed samples from patients and made a genetic change to boost the amount of fetal hemoglobin in their red blood cells. These stem cells are reinfused into the patient. According to New Atlas, “the first two patients treated were essentially cured within months, but questions over long-term efficacy remained.” In a follow-up, the scientists reported that 22 patients demonstrating complete success, and of those, seven patients continued to see the same level of efficacy from the treatment.
In their report, the scientists noted that 42 out of 44 patients with transfusion-dependent beta thalassemia were cured to the point that they no longer require blood transfusion treatments. Two of the patients did still need to receive blood transfusions, but they had 75% and 89% reductions in how much they needed to be transfused, which are promising results, to be sure.
The information presented at the European Hematology Association Congress on this work with CRISPR is not yet published in a peer-reviewed scientific journal, so more work is needed, along with examination by other scientists to check on the validity of the results so far.
But with good results in so many patients as reported EHAC, it looks like patients with the rare blood diseases may eventually find a true cure that keeps them from needing to undergo blood transfusions to lead a healthier life.
Potential Approval for Public Use of Exa-Cel in the United States
Keep in mind that medical research can be a slow and painstaking process. It tends to involve multiple clinical trials, with oversight from government regulators along with other scientists evaluating work as reported in peer-reviewed journals. The market research can be done via specialized market research companies.
In the case of exa-cell, New Atas reports that the United States Food and Drug Administration has approved Fast Track designation for this therapy. Vertex indicated it wants to submit exa-cel for market approval to the FDA by the end of 2022.
If the FDA does authorize exa-cel, it will be the first time the U.S. has approved of a treatment based on CRISPR gene editing. So, to stay on top of developments in FDA oversight on exa-cel and its potential use in the coming year, stay tuned to this space for updates.